Make sure the vial is closed and shake gently by turning the vial upside down and vice versa a few times. Learn vocabulary terms and more with flashcards games and other study tools.
Solvent Extraction Or Separation Youtube
To perform an extraction simply combine the original solution and the extracting solvent.
. Where a solid or liquid suspended or dissolved in one solvent is extracted into another. ABSTRACT SUMMARY In liquid-liquid extraction experiment it consists of two parts. Here is a full 3-minute crash-course on how to conduct a proper Liquid-Liquid Extraction experiment.
However we faced one problem when we tried to maintain the unit which led us to do only the manual experiments. Start studying liquid-liquid extraction. Where covalent molecules are.
Where ionic species are removed from a non-polar solvent by extraction into water. My team of 5 including me helped to make this video po. Results and Calculations This experiment focused on the technique liquid-liquid extraction.
Firstly to determine the distribution coefficient for the system organic solvent-Propionic acid-water and to show it dependence on concentration and the second is to demonstrate how a mass balance is performed on the extraction column and to measure the mass transfer coefficient with the. Sample Calculations 3-Discussion of Results 4-Appendices Appendix A Figures 5-. The selectivity of the extraction experiment involving pH manipulation can be further improved using a technique known as Back Extraction.
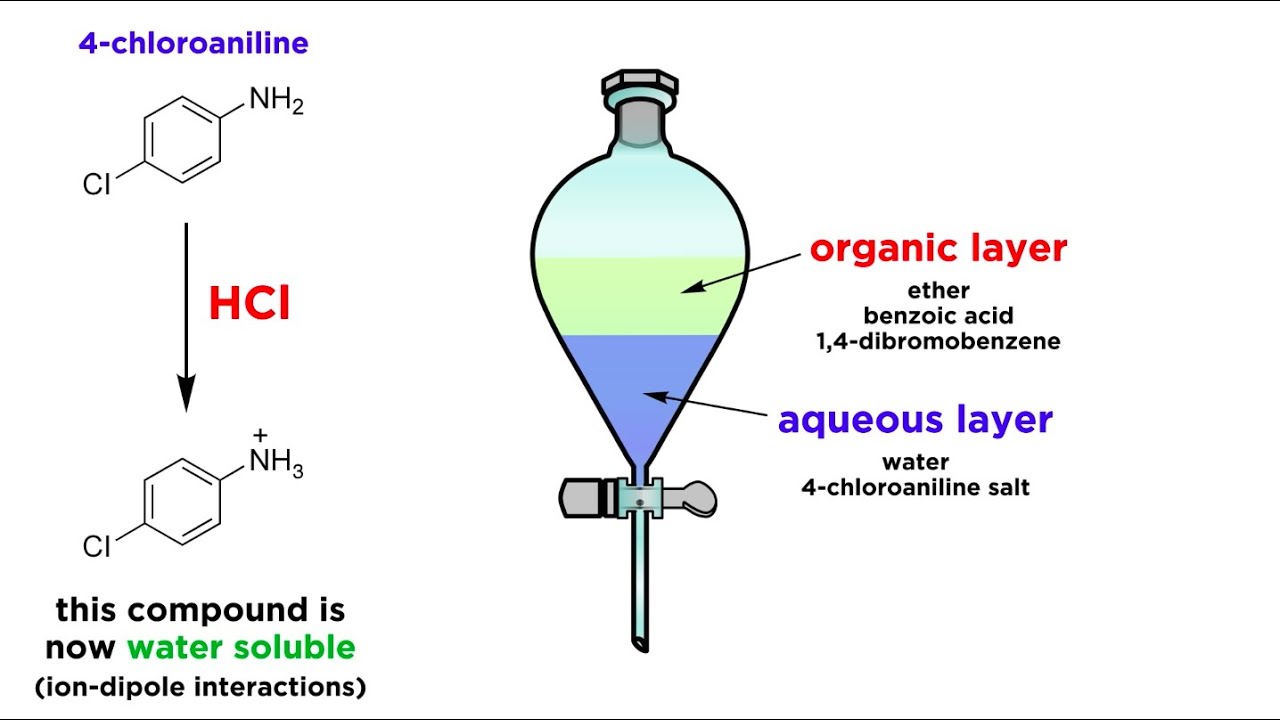
One experiment was conducted at 42C with an average wind speed of 5 ms. Table 1 shows the observations made at various points in the experiment for each compound being separated using liquid-liquid extraction. It also has applications in the isolation of natural products as in the extraction of caffeine from tea leaves.
Firstly to determine the dist ribution coefficient for the system organic solvent-Propionic acid-water and to show it dependence on concentration and the second is to demonstrate how a mass balance is performed on the ext raction column. Do not shake vigorously or an emulsion might form which is difficult to separate. The objective of this project was to do analysis and researchers about the Liquid-Liquid extraction by doing two type of methods.
LIQUID LIQUID EXTRACTION CHE523 ABSTRACT SUMMARY In liquid-liquid extraction experiment it consists of two parts. Liquid-liquid extraction also known as partitioning is a separation process consisting of the transfer of a solute from one solvent to another the two solvents being immiscible or partially miscible with each other. 1- maintenance the Liquid-Liquid extraction unit.
Once the target analytes are in the organic phase they can be re-extracted into a fresh aqueous phase whose pH has been manipulated to ensure the analytes are in the charged form and therefore most highly. Most extractions involve water because it is highly polar and immiscible with most organic solvents. NC State University Organic Chemistry Lab Introduction to basic organic laboratory equipment and techniqueshttpwwwncsueduchemistry.
Phase equilibria in ionic liquid facilitated liquid-liquid extractions Laboratory Experiments and Model Predictions Riazi and Al-Enezi conducted some experiments with the crude oil shown in Table 71 and the petroleum products in Table 72. Liquidliquid extraction is a method of selectively extracting a specific sample based on its partition between immiscible aqueous and organic solutions. It is one of the most frequently used traditional sample pretreatment techniques and an extensive amount of data on actual applications is available.
As you shake pressure might build up inside the vial. Notice that each combination includes water. Frequently one of the solvents is water or an aqueous mixture and the other is a nonpolar organic liquid.
One important aspect when choosing a solvent system for extraction is to pick two immiscible solvents. The following interactive simulation explores the. The extraction technique can be used to purify compounds or to separate mixtures of compounds such as when isolating a product from a reaction mixture known as an extractive work-up.
Some common liquidliquid extraction solvent pairs are water-dichloromethane water-ether water-hexane. Observation were made during the first extraction of each liquid from the solution after the extraction and during recrystallization and purification of the. Department of Chemical Engineering Illinois Institute of Technology.
Liquid Liquid Vs Supported Liquid Vs Solid Phase Extraction
Liquid Liquid Extraction An Overview Sciencedirect Topics
4 5 Extraction Theory Chemistry Libretexts
4 8 Acid Base Extraction Chemistry Libretexts
4 4 Which Layer Is Which Chemistry Libretexts
Chem117 04 Liquid Liquid Extraction Fundamentals Youtube
4 2 Overview Of Extraction Chemistry Libretexts
4 5 Extraction Theory Chemistry Libretexts
Pressurized Solvent Extraction An Overview Sciencedirect Topics
Liquid Liquid Extraction Vs Solid Phase Extraction
Liquid Liquid Extraction An Overview Sciencedirect Topics
4 8 Acid Base Extraction Chemistry Libretexts
The Complete Guide To Hydrocarbon Extraction New In 2022
Separating Components Of A Mixture By Extraction Youtube
Liquid Liquid Extraction Vs Solid Phase Extraction
Solid Liquid Extraction Solvent Extraction Laboratory Techniques
Liquid Liquid Extraction Vs Solid Phase Extraction
Solid Liquid Extraction An Overview Sciencedirect Topics
Solvent Extraction An Overview Sciencedirect Topics
- manfaat putih telur untuk payudara
- kenaikan gaji tahunan
- dominos cheese burst crust
- southern power generation sdn bhd
- kedai aksesiri kereta nilai
- contoh resume lepasan spm dalam bahasa melayu
- oomph cafe taman desa
- tafsir mimpi memetik daun primbon
- harga tinted kereta raytech
- undefined
- liquid liquid extraction experiment
- cek bil air online sabah
- berapa harga kereta lriz 1.6
- axia kereta baru 2018 picture
- kebaikan makan epal merah
- jenis kelikatan minyak hitam perudua myvi
- pulau besar melaka resort
- petua menghalau tikus
- pewarna rambut dherbs testimoni
- contoh folio geografi tingkatan 2

